FAQs
-
What is a battery?
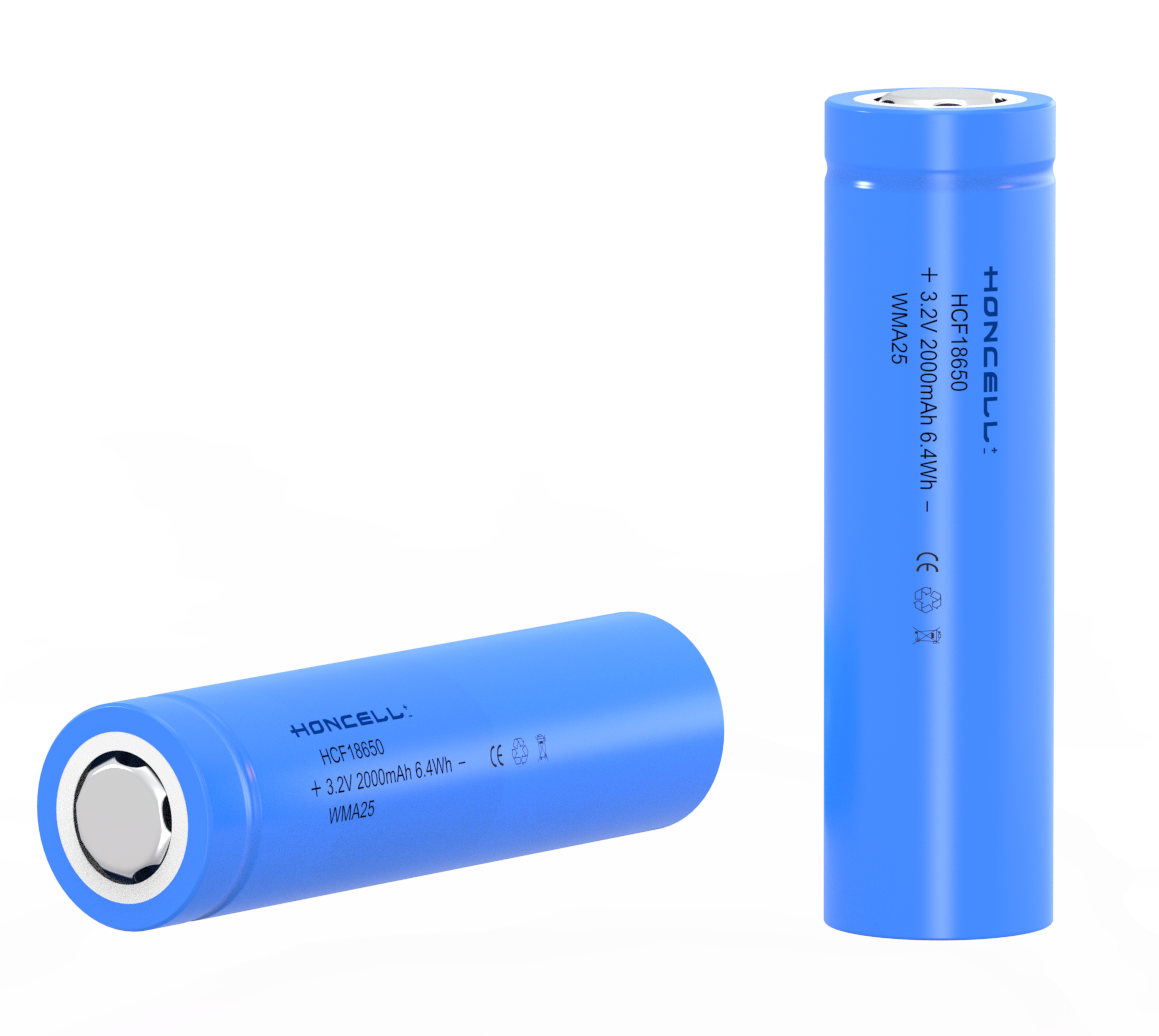
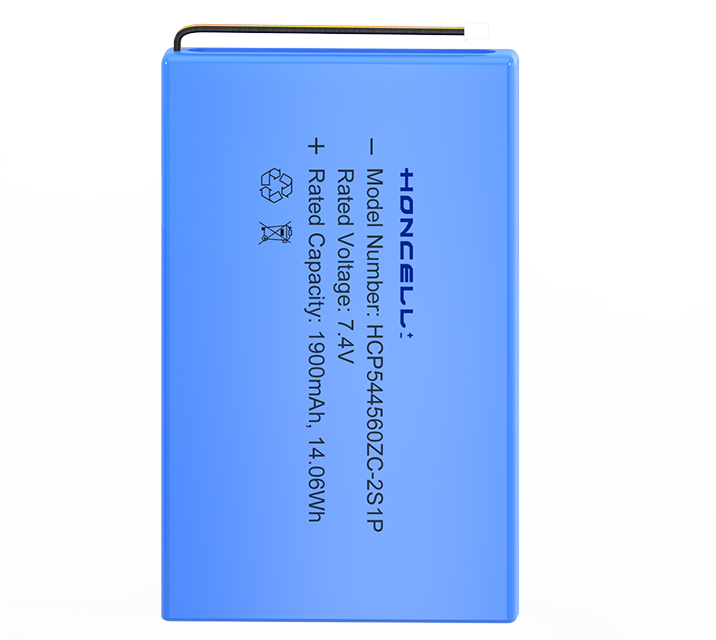
A battery, in concept, can be any device that stores energy for later use. A rock, pushed to the top of a hill, can be considered a kind of battery, since the energy used to push it up the hill (chemical energy, from muscles or combustion engines) is converted and stored as potential kinetic energy at the top of the hill. Later, that energy is released as kinetic and thermal energy when the rock rolls down the hill. Common use of the word, "battery," however, is limited to an electrochemical device that converts chemical energy into electricity, by use of a galvanic cell. A galvanic cell is a fairly simple device consisting of two electrodes (an anode and a cathode) and an electrolyte solution. A battery is an electrical storage device. Batteries do not make electricity, they store it, just as water tank stores water for future use. As chemicals in the battery change, electrical energy is stored or released. In rechargeable batteries this process can be repeated many times. Batteries are not 100% efficient - some energy is lost as heat and chemical reactions when charging and discharging.